MediGrid
Make your research data findable, accessible, interoperable and reusable.
Where is your research data stored after a study has finished? Somewhere on a network drive? Or in some old application most people don’t use anymore? In countless Excel files? You are not the only one.
We believe in proper medical data management across the entire data lifecycle. By properly storing, organizing and describing your data it becomes much more valuable to your organization. This is also the main enabler for applications such as AI, data sharing and meta-analysis. At the same time you will be more in control of your data, resulting in better security, compliance and access control.
50% of CROs is still using paper to exchange information with sites
Make your clinical research data FAIR
FAIR stands for Findable, Accessible, Interoperable and Reusable. It is a set of principles that promote well governed data, open science and active data sharing. FAIR is fast becoming the norm in the research industry. Not only in health, clinical or biotech, but across broader academia as well.
Create more insights from your data
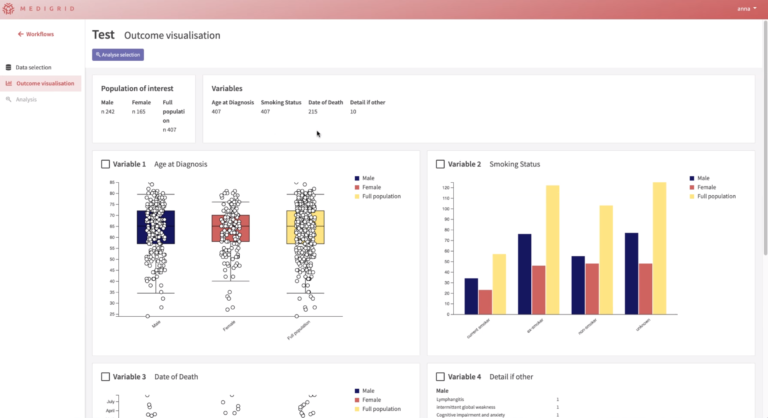
Most researchers and analysts are perfectly able to analyze a dataset using tools such as Excel, SPSS, SAS, R etc. It becomes much more challenging when you want to harmonize your data across different data sets. Or want to work consistently with standardized terminologies or data dictionaries.
MediGrid has a smart data ingestion engine that not only structures your data well and helps to curate your data, it is also able to transform and harmonize your data. This allows researchers to conduct multi-study analyses or to review adverse effects across multiple studies for example.
Detect and manage safety risks
During various phases of your research you want a realtime view of the safety of all patients. Especially when it comes to monitoring of adverse effects (AE) and serious adverse events (SAE) before or after market introduction. MediGrid can help you monitor, detect and warn you about these safety risks. This will improve patient safety and prevent you from getting a bad reputation. MediGrid also does the heavy lifting in terms of collecting, classifying, harmonizing and reporting the safety data.


Your first FAIR data catalog live in 4 hours
Our platform and smart ingestion engine help you set up your environment, define your data model, import your data, do your first analysis and setup your first FAIR data catalog all in less than 4 hours. No projects of several months, no waiting for IT resources, just fast & easy. That’s our promise.
The best way to learn more
There is lots more to tell en learn. The best way to learn more about MediGrid is by experiencing a demo. In only 30 minutes you’ll learn more about the solution, see the interface and several of the analysis options.
